WATER BASED MUD
WATER BASED MUD
Water itself may be used as a drilling fluid. However, most drilling fluids require some degree of viscosity to suspend the Barites and to carry drilled cuttings up the annulus of the wellbore. The viscosity of water based muds is generated by the addition of clay or polymers. However the cheapest and most widely used additive for viscosity control is clay. The clay material in water based mud is responsible for two beneficial effects:
• An increase in viscosity which improves the lifting capacity of the mud to carry cuttings to the surface. (This is especially helpful in larger holes where annular velocity is low).
• Building a wall cake in permeable zones, thus preventing fluid loss.
The clays are not the only solids in a drilling fluid. There are two types of solids
which may be present in a water based mud:
• Active solid — these are solids which will react with water and can be controlled by chemical treatment. These may be commercial clays or hydratable clays from the formations being drilled.
• Inactive or inert solids — these are solids which do not readily react with water. These may be drill solids such as limestone or sand. Barite is also an inert solid.
In order to appreciate how clays play an important part in water based muds some understanding of clay chemistry is necessary.
3.1 Clay Chemistry (See Appendix 1)
Clay minerals can be divided into two broad groups.
• Expandable (hydrophyllic) clays — these will readily absorb water (e. g. montmorillonite).
• Non-expandable (hydrophobic) clays — these will not readily absorb water (e. g. illite).
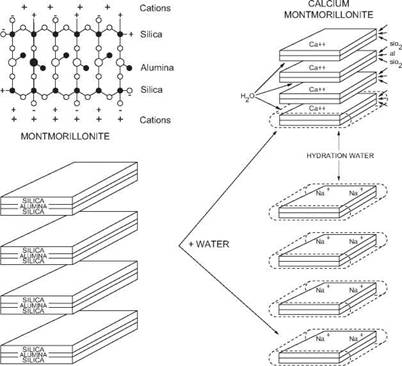
Clay minerals have a sandwich-like structure usually consisting of three layers. The alternate layers are of silica and alumina. A clay particle usually consists of several sandwiches stacked together like a pack of cards.
|
SODIUM or CALCIUM SODIUM MONTMORILLONITE MONTMORILLONITE |
Figure 12 Hydration of Montmorillonite
Expandable and Non-expandable clays in water
The most commonly clay used in drilling fluids is Wyoming Bentonite (sodium montmorillonite). Figure 12 shows a simplified diagram of its structure. In fresh water the clay layers absorb water, the chemical bonds holding them together are weakened and the stack of layers disintegrates. This process is known as dispersion (i. e. less face-to-face association). Dispersion results in an increase in the number of particles in suspension, which in turn increases the number of suspended particles and causes the fluid to thicken or viscosify. During this process, positively charged cations separate from the clay surface leaving the flat surface of the particles negatively charged while the edges are positively charged. It is likely therefore that some plates will tend to form edge-to-face arrangements. This process is known as flocculation.
In a Bingham Plastic fluid, Plastic viscosity can be thought of as that part of the flow resistance caused by mechanical friction between the particles present in the mud and will therefore be dependant on solids content. Yield point is that component of resistance caused by electro-chemical attraction within the mud while it is flowing.
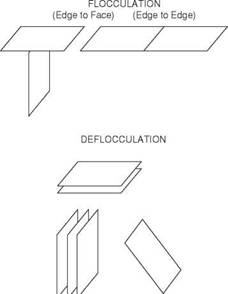
There are 4 arrangements of clay particles which are commonly encountered (Figure 13):
AGGREGATION (Face to Face)
|
|
DISPERSION
Figure 13 Association of clay particles
Aggregation (Face-to-face) is the natural state for the clay particles. In this configuration there are a small number of particles in suspension and therefore the plastic viscosity of the mud is low. If the mud has, at some time been dispersed, aggregation may be achieved by introducing cations (e. g. Ca2+) to bring the plates together. Lime or gypsum may be added to achieve this effect.
Dispersion occurs when the individual clay platelets are dispersed by some mechanism. Dispersion increases the number of particles and causes an increase in plastic viscosity. Clays will naturally disperse in the presence of freshwater but this process will be enhanced by agitation of the mud. Bentonite does not usually completely disperse in water.
Flocculation is when a house of cards structure is formed because of the attraction between the positive charges on the face of the particles and the negative charges on the edge of the particles. Flocculation increases the viscosity and yield point of the mud. The severity of flocculation depends on the proximity of the charges acting on the linked particles. Anything that shrinks the absorbed water film around the particles (e. g. temperature) will decrease the distance between the charges on the particles and increase flocculation.
De-flocculation occurs when the house of cards structure is broken down and something is introduced into the mud that reduces the edge-to-face effect. Chemicals called “thinners’ are added to the mud to achieve this.
a. Viscosity control additives
Commercial clays are used to control the viscosity of water based muds. These are graded according to their yield. The yield of a clay is defined as the number
of barrels of 15 centipoise viscosity mud which can be obtained from 1 ton of dry clay. (A 15 centipoise viscosity will support barite). Wyoming bentonite has a yield of about 100 bbl/ton, whereas native clays may only yield 10 bbl/ton. The result of this would be that the native clay would cause a higher solids content and mud density than the Wyoming bentonite to build the same viscosity. The specifications for bentonite are laid down by the API and are shown in Table 4. The yield of a clay will be affected by the salt concentration in the mixwater. The hydration and therefore dispersion of the clay are greatly reduced by the presence of Ca2+ and Mg2+ ions. To overcome this problem various measures can be taken:
• Chemical treatment to reduce salt concentration by precipitation.
• Dilution with fresh water.
• Attapulgite clay may be used. Attapulgite has a different structure to montmorillonite and does not depend on the type of make up water to build viscosity. It is however more expensive and provides poor filtration control.
• first hydrate the clay in fresh water, then add the slurry to the salt water.
• use organic polymers (cellulose) to build viscosity.
|
Type of solid |
meq/100g of solids |
|
Attapulgite clay |
15 — 25 |
|
Chlorite clay |
10 — 40 |
|
Gumbo shale |
20 — 40 |
|
Illite clay |
10 — 40 |
|
Kaoline clay |
3 — 15 |
|
Montmorillonite clay |
80 — 150 |
|
Sandstone |
0 — 5 |
|
Shale |
0 — 20 |
|
Table 3 Methyline Blue Absorptive Capacity |
|
600 rpm viscometer reading: |
30 cp (min.) |
|
YP: |
3 x PV (min.) |
|
Filtrate: |
13.5 ml (max.) |
|
Residue on No. 200 sieve: |
2.5% (max.) |
|
Moisture content: |
10% (max.) |
|
Yield: |
91.8 bbls/ton |
|
Table 4 API Specification for Bentonite |
|
Specific gravity: |
4.2 (min) |
|
Soluble metals or calcium: |
250 ppm (max) |
|
Wet screen analysis |
|
|
Residue on No. |
200 sieve: 3% (max) |
|
Residue on No. |
325 sieve: 5% (min) |
|
Table 5 API Specification for Barite |
To reduce the viscosity of the mud:
• Lower the solids content.
• Reduce the number of particles per unit volume.
• Neutralize attractive forces between the particles.
The use of screens, desilters and other mechanical devices will reduce viscosity, but chemical additives may also be used. These chemicals produce negatively charged anions in solution and thereby reduce the positive charge on the edge of the clay plates. This reduces the edge-to-face association and therefore reduces viscosity. Such chemicals are called thinners (or dispersants) and include: Phosphates;
Lignites; Lignosulphonates; and Tannins.
b) Density control additives
Barite (barium sulphate, BaSO4) is the primary weighting material used in muds. Densities of 9 ppg to 19 ppg can be achieved by mixing water, clay and barite. The API specification for barite is shown in Table 5. Other weighting materials are calcium carbonate and galena (lead sulphide). The drill solids from the formation will increase the mud density if they are not separated out. This will be discussed under solids control.
c) Filtration control additives
Loss of fluid from the mud occurs when the mud comes into contact with a permeable zone. If the pores are large enough the first effect will be a spurt loss, followed by the buildup of solids to form a mud cake. The rate at which fluid is lost is a function of the differential pressure, thickness of filter cake and viscosity of the filtrate. Excessive filtration rates and thick wall cake can lead to problems such as:
• Tight spots in the hole
• Differential pipe sticking
• Formation damage due to filtrate invasion
Since a filter cake attains its greatest thickness under static conditions the mud is tested under static conditions. Dynamic filtration results in a thinner mud cake due to erosion effects, but the rate of filtration will be higher. The aim is to deposit a thin and impermeable filter cake. Several types of material may be added to the mud to control fluid loss.
• Clays — Bentonite is an effective fluid loss control agent because of its particle size and shape, and also because it hydrates and compresses under pressure. The particle size distribution is such that most particles will be less than 1 micron.
Care should be taken not to remove these small particles by using a centrifuge for solids control.
• Starch — These organic chemicals will swell rapidly and seal off the permeable zones effectively.
• CMC — This is an organic colloid (sodium carboxyl-methyl cellulose). The long chain molecules can be polymerized into 3 different grades (high, medium and low viscosity). It is thought that CMC controls filtration by wedging long chain polymers into the formation and plugging the pores. CMC works well in most water-based muds, but less effective in high salt concentrations (>50,000 ppm).
• Polyacrylates (Cypan) — These are long chain polymers which become absorbed onto the edge of clay particles.
• Lignosuphonates — Similar in action to starch in reducing fluid loss.
• Polyanoinic cellulose (Drispac) — An organic compound which is used to control fluid loss in high salt concentrations, and is often used in low solids mud. May also be used as a viscosifier.
The water loss allowable in any particular area will largely depend on experience. As the well is being drilled the fluid loss must be adjusted as new formations are penetrated. The surface hole may be drilled with a fluid loss of 20 cc, but across productive formations it will be reduced (down to possibly 5 cc). Control over fluid loss depends on the correct addition of chemicals and keeping the clay solids dispersed. Fluid loss control agents may also act as thinners, or viscosifiers under certain circumstances, and react unfavourably with other chemicals in the mud.
d) pH control additives
Caustic soda NaOH is the major additive used to keep the pH of the mud high. This is desirable to prevent corrosion and hydrogen embrittlement. The pH of most muds lies between 9.5 and 10.5. Caustic potash, KOH and slaked lime, Ca(OH)2 may also be used.
e) Removal of contaminants
Various substances may enter the mud and cause an adverse effect on the quality of the mud and reduce its efficiency. These contaminates must be removed. The main contaminates are listed below:
• Calcium (Ca2+) — may enter from cement, gypsum, lime or saltwater. It reduces the viscosity building properties of bentonite. It is usually removed from fresh water muds by adding soda ash Na2CO3, which forms insoluble CaCO3. If calcium is present in the mud the pH will normally be too high.
• Carbon dioxide (CO2) — present in formations which when entrained in the mud can cause adverse filtration and gelation characteristics. To remove CO2 calcium hydroxide can be added to precipitate CaCO3.
|
|
• Hydrogen sulphide (H2S) — present in formations. Highly toxic gas which also causes hydrogen embrittlement of steel pipe. Add NaOH to keep pH high and form sodium sulphide. If the pH is allowed to drop the sulphide reverts back to H2S
• Oxygen (O2) — entrained into mud in surface pits, causes corrosion and pitting of steel pipe. Sodium sulphite (Na2SO3) is added at surface to remove the Oxygen.
3.3 Special Types of Water Based Muds
The hydration of clays is severely reduced if the water used to make up the mud contains a high salt concentration. If a shale zone is being drilled with a freshwater mud the clays in the formation will tend to expand and the wellbore becomes unstable (sloughing shale). By using a mud containing salt or calcium there will be less tendency for this problem to occur. An inhibitive mud is defined as one where the ability of active clays to hydrate has been greatly reduced. Another advantage is that the water normally used in hydration is available to carry more solids. Inhibitive muds are principally used to drill shale and clay formations, and are characterised by:
• Low viscosity
• Low gel strength
• Greater solids tolerance
• Greater resistance to contaminants
a. Calcium treated muds
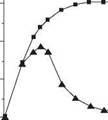
When Ca2+ ions are added to a clay-water mud the mud begins to thicken due to flocculation. At the same time a cation exchange reaction begins whereby Ca2+ replaces Na2+ on the clay plates. Calcium montmorillonite does not hydrate as extensively as sodium montmorillonite, and the plates begin to aggregate. As the reaction proceeds the mud begins to thin and viscosity reduces.
|
100
о ^ 60 |
120
|
|
|
40 |
High Solids
-A— Low Solids
20
|
0 |
|
200 |
|
600 |
|
800 |
|
400 |
Filtrate Calcium
The conversion of a fresh water mud to an inhibited mud usually takes place in the wellbore. The conversion should not be done at a shallow depth where large volumes of cuttings are being lifted, as this might cause a viscous plastic mass around the bit. Figure 14 shows how the viscosity varies during this conversion. Gypsum CaSO4.2H2O or calcium chloride CaCl2 can be used in place of lime to supply the Ca2+ ions.
b. Lignosulphanate treated muds
An inhibited mud can also be formed by adding large amounts (12 lb/bbl) of lignosulphanate to a clay-water system. Chrome lignosulphanate is commonly used since it is relatively cheap and has a high tolerance for salt and calcium.
c. Saltwater muds
Inhibitive muds having a salt concentration (NaCl) in excess of 1% by weight are called salt water muds. These are often used in marine areas where fresh water is not readily available. As stated earlier commercial clays (e. g. bentonite) will not readily hydrate in water containing salt concentration (i. e. bentonite behaves like an inert solid). To build viscosity therefore the clay must be prehydrated in fresh water, then treated with deflocculant before increasing salinity. The Ca2+ and Mg2+ions can be removed by adding NaOH to form insoluble precipitates which can be removed before building viscosity. After conversion salt water muds are not greatly affected by subsequent contamination. However the increased salt content may make it more difficult to maintain other mud properties. (Alkalinity is controlled by adding NaOH and filtration by adding bentonite). Corrosion may be a major problem in salt water muds unless alkalinity is controlled.
d. KCL — polymer system
This mud system was specifically developed to combat the problem of water sensitive, sloughing shales. The potassium chloride concentration must be at least 3 — 5% by weight to prevent swelling of shales containing illite and kaolinite. For shales containing bentonite the KCl concentration must be raised to 10%. Polyacrylamide polymers are used to control the viscosity of the mud and are used in concentrations of around 0.75 lb/bbl. Potassium hydroxide or caustic soda may be used to control the pH at around 10. This system allows good shale stabilisation, hole cleaning and flocculation of drilled solids. The KCl polymer system is stable up to 300 degrees F. Temperatures above 300 degrees F will cause slow degradation of the polymer.
e. Polyol muds
Polyol is the generic name for a wide class of chemicals — including glycerol, polyglycerol or glycols such as propylene glycol — that are usually used in conj unction with an encapsulating polymer (PHPA) and an inhibitive brine phase (KCl). These materials are nontoxic and pass the current environmental protocols, including those laid down in Norway, the UK, The Netherlands, Denmark and the USA.
Glycols in mud were proposed as lubricants and shale inhibitors as early as the 1960s. But it was not until the late 1980s that the materials became widely considered. Properly engineered polyol muds are robust, highly inhibitive and often cost-effective. Compared with other WBM systems, low volumes of additives are typically required. Polyols have a number of different effects, such as lubricating the drillstring, opposing bit balling (where clays adhere to the bit) and improving
fluid loss. Today, it is their shale-inhibiting properties that attract most attention. For example, tests carried out by BP show that the addition of 3 to 5% by volume of polyglycol to a KCl-PHPA mud dramatically improves shale stabilization. However, a significant gap still remains between the performance of polyol muds and that of OBM.
Field experience using polyol muds has shown improved wellbore stability and yielded cuttings that are harder and drier than those usually associated with WBM. This hardness reduces breakdown of cuttings and makes solids control more efficient. Therefore, mud dilution rates tend to be lower with polyol muds compared with other WBM systems (for an explanation of solids control and dilution, see mud management).
As yet, no complete explanation of how polyols inhibit shale reactivity has been advanced, but there are some clues:
• Most polyols function best in combination with a specific inhibitive salt, such as potassium, rather than nonspecific high salinity.
• Polyol is not depleted rapidly from the mud even when reactive shales are drilled.
• Many polyols work effectively at concentrations as low as 3%, which is too low to significantly change the water activity of the base fluid.
• Polyols that are insoluble in water are significantly less inhibitive than those that are fully soluble.
• No direct link exists between the performance of a polyol as a shale inhibitor and its ability to reduce fluid loss.
Many of these clues eliminate theories that try to explain how polyols inhibit shales. Perhaps the most likely hypothesis — although so far there is no direct experimental evidence supporting it — is that polyols act as a structure breaker, disrupting the ordering of water on the clay surface that would otherwise cause swelling and dispersion. This mechanism does not require the glycol to be strongly absorbed onto the shale, which is consistent with the low depletion rates seen in the field.
f. Mixed-metal hydroxide (MMH) mud
MMH mud has a low environmental impact and has been used extensively around the world in many situations: horizontal and short-radius wells, unconsolidated or depleted sandstone, high-temperature, unstable shales, and wells with severe lost circulation. Its principal benefit is excellent hole-cleaning properties.
Many new mud systems — including polyol muds — are extensions of existing fluids, with perhaps a few improved chemicals added. However, MMH mud is a complete departure from existing technology. It is based on an insoluble, inorganic, crystalline compound containing two or more metals in a hydroxide lattice — usually mixed aluminium/magnesium hydroxide, which is oxygen-deficient. When added to prehydrated bentonite, the positively charged MMH particles interact with the negatively charged clays forming a strong complex that behaves like an elastic solid when at rest. This gives the fluid its unusual rheology: an exceptionally low plastic viscosity-yield point ratio. Conventional muds with high gel strength usually require high energy to initiate circulation, generating pressure surges in the annulus once flow has been established. Although MMH has great gel strength at rest, the structure is easily broken. So it can be transformed into a low-viscosity fluid that does not induce significant friction losses during circulation and gives good hole cleaning at low pump rates even in high-angle wells. Yet within microseconds of the pumps being turned off, high gel strength develops, preventing solids from settling.
There are some indications that MMH also provides chemical shale inhibition. This effect is difficult to demonstrate in the laboratory, but there is evidence that a static layer of mud forms adjacent to the rock face and helps prevent mechanical damage to the formation caused by fast-flowing mud and cuttings, controlling washouts.
MMH is a special fluid, sensitive to many traditional mud additives and some drilling contaminants. It therefore benefits from the careful management that is vital for all types of drilling fluid.
g. Silicate Fluids
Silicate is used as a shale hydration suppressor. The Sodium Silicate precipitates a layer of Silicate over the reactive sites on the clay particle and over microfractures in the matrix thus preventing hydration by water migration into the clay.