 Engine power output using producer gas
Engine power output using producer gas
The power output from an engine operating on producer gas will be determined by the same factors as for engines operating on liquid fuels, namely:
— the heating value of the combustible mixture of fuel and air which enters the engine during each combustion stroke;
— the amount of combustible mixture which enters the engine during each combustion stroke;
— the efficiency with which the engine converts the thermal of the combustible mixture into mechanical energy (shaft power);
— the number of combustion strokes in a given time (number of revolutions per minute: rpm);
Conversion of an engine to producer gas or dual-fuel operation will generally lead to a reduced power output. The reasons for this and possibilities to minimize the power loss will be discussed below.
(a) Heating value of the mixture
The heating value of producer gas depends on the relative amounts of the different combustible components: carbon monoxide, hydrogen and methane.
The heating value of these three gases are given in Table 2.1.
|
Table 2.1. Heating values and stoichiometric oxygen demands of combustible producer gas components.
1/ The gas volume is given as normal — m, unless otherwise specified, throughout the publication. |
In order to achieve combustion however, the producer gas has to be mixed with a suitable amount of air. The combustible mixture will have a lower heating value per unit volume than producer gas alone.
The amounts of oxygen necessary for complete burning (stoichiometric combustion) of each of the combustible components are also presented in Table 2.1.
The heating value of such a stoichiometric mixture can be calculated from the following formula:
12680 V„ + 10800 Vh, + 35900 Hig = 1 + 2.38 V„ + 238 VHi + 9.52
where:
Hig — is the heating value of a stoichiometric mixture of producer gas and air in kJ/m3 VCo — volume fraction of carbon monoxide in the gas (before mixing with air)
— volume fraction of hydrogen in the gas (before mixing with air)
VcH* — volume fraction of methane in the gas (before 4 mixing with air).
Heating values of producer gas and air mixtures are around 2500 kJ/m3. When this value is compared with the heating value of a stoichiometric mixture of petrol and air (about 3800 kJ/m3), the difference in power output between a given engine fuelled by petrol and by producer gas becomes apparent. A power loss of about 35% can be expected as a result of the lower heating value of a producer gas/air mixture.
(b) Amount of combustible mixture supplied to the cylinder
The amount of combustible mixture which actually enters the cylinder of an engine is determined by the cylinder volume and the pressure of the gas in the cylinder at the moment the inlet valve closes.
The cylinder volume is a constant for a given engine. The actual pressure of the combustible mixture at the start of the compression stroke depends however on engine characteristics (especially the design of inlet manifold and air inlet gate), the speed of the engine (higher speeds tend to result in lower pressures), and on the pressure of the gas entering the air inlet manifold. The former two factors are incorporated in the so called "volumetric efficiency" of the engine, which is defined as the ratio between the actual pressure of the gas in the cylinder and normal pressure (1 atm). Normally engines running at design speeds show volumetric efficiencies varying between 0.7 and 0.9.
The pressure of the gas at the air inlet manifold depends on the pressure drop over the total gasification system, i. e. gasifier cooler/cleaner, and gas/air carburettor. This drop reduces again the entering pressure by a factor of 0.9.
In sum, it must be concluded that the actual amount of combustible gas available in the cylinder will be only 0.65 — 0.8 times the theoretical maximum because of pressure losses on the way to the cylinder. This will obviously reduce the maximum power output of the engine.
(c) Engine efficiency
The efficiency with which an engine can convert the thermal energy in the fuel into mechanical (shaft) power, depends in the first instance on the compression ratio of the engine.
The influence of increasing the compression ratio of an engine can be calculated from the following formula.
T)i_T)o= ^ k_s0 k
In which:
ц і = engine thermal efficiency at compression ratio г) о = engine thermal efficiency at compression ratio є і = engine compression ratio in situation 1 є о = engine compression ratio in situation 0 к = a constant equal to 1.3 in the case of producer gas
Figure 2.2 Relation between compression ratio and thermal efficiency of an engine (7)
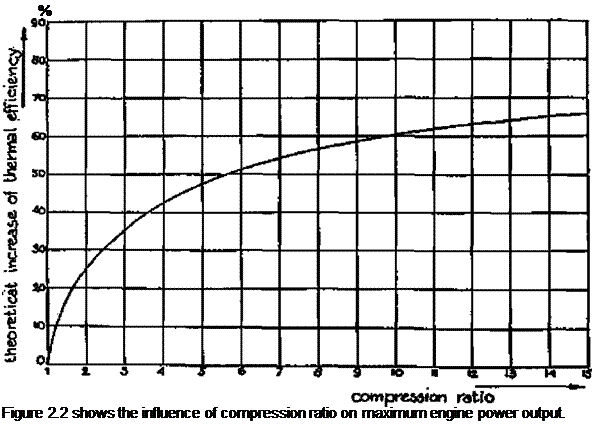 |
Figure 2.2 Relation between compression ratio and thermal efficiency of an engine
(34)
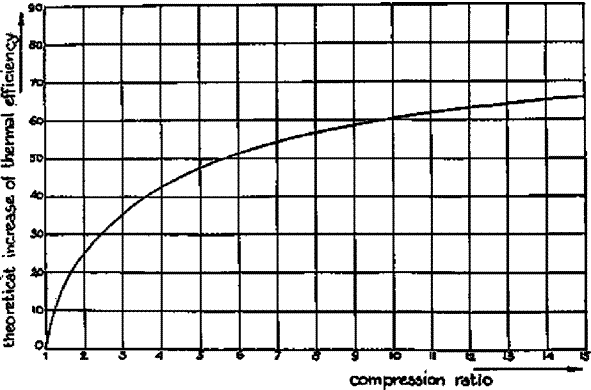 |
In the case of engines fuelled by petrol, the possible compression ratio is limited by the "octane" number of the fuel, which is a measure of the compression ratio at which detonation or "knocking" (which can lead to severe engine damage) occurs. Producer gas/air mixtures show higher octane numbers than petrol/air mixtures.
It is for this reason that higher compression ratios (up to 1:11) can be employed with producer gas, resulting in better engine thermal efficiencies and a relative increase in engine shaft power output.
(d) Engine speed
Because the engine power output is defined per unit time, the engine power output depends on the engine speed.
For diesel engines the power output is nearly linear with the rpm. For spark ignition engines the power increase is less than linear because of changes in the different efficiency factors.
When the power output of a 4-stroke engine is calculated, allowance must be made for the fact that only one out of every two rotations represents a compression and combustion stroke.
The maximum speed of engines fuelled by producer gas is limited by the combustion velocity of the combustible mixture of producer gas and air. Because this speed is low as compared to combustible mixtures of petrol and air, the efficiency of the engine can drop dramatically if the combustion speed of the mixture and the average speed of the piston become of the same order of magnitude.
In the types of engines that are currently mass-produced, one can expect this phenomenom to occur at engine speeds of around 2500 rpm. Engines fuelled by producer gas should therefore generally be operated below this speed.