 Theory of gasification
Theory of gasification
The substance of a solid fuel is usually composed of the elements carbon, hydrogen and oxygen. In addition there may be nitrogen and sulphur, but since these are present only in small quantities they will be disregarded in the following discussion.
In the types of gasifiers considered here, the solid fuel is heated by combustion of a part of the fuel. The combustion gases are then reduced by being passed through a bed of fuel at high temperature.
In complete combustion, carbon dioxide is obtained from the carbon and water from the hydrogen. Oxygen from the fuel will of course be incorporated in the combustion products, thereby decreasing the amount of combustion air needed.
Oxidation, or combustion, is described by the following chemical reaction formulae:
C+O2«CO2_401 g kJ/m0|
H+1/202^H20_2411 kJ/mol
These formulae mean that burning 1 gram atom, i. e. 12.00 g of carbon, to dioxide, a heat quantity of 401.9 kJ is released, and that a heat quantity of 241.1 kJ results from the oxidation of 1 gram molecule, i. e. 2.016 g of hydrogen to water vapour.
In all types of gasifiers, the carbon dioxide (C02) and water vapour (H20) are converted (reduced) as much as possible to carbon monoxide, hydrogen and methane, which are the main combustible components of producer gas.
The most important reactions that take place in the reduction zone of a gasifier between the different gaseous and solid reactants are given below. A minus sign indicates that heat is generated in the reaction, a positive sign that the reaction requires heat.
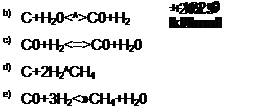 |
a) C+ C02 «2СО + 164 9 kJ/kmol
Equations (a) and (b), which are the main reactions of reduction, show that reduction requires heat. Therefore the gas temperature will decrease during reduction.
Reaction (c) describes the so-called water-gas equilibrium. For each temperature, in theory, the ratio between the product of the concentration of carbon monoxide (CO) and water vapour (H20) and the product of the concentrations of carbon dioxide (C02) and hydrogen (H2) is fixed by the value of the water gas equilibrium constant (KWe)- In practice, the equilibrium composition of the gas will only be reached in cases where the reaction rate and the time for reaction are sufficient.
The reaction rate decreases with falling temperature. In the case of the water-gas equilibrium, the reaction rate becomes so low below 700°C that the equilibrium is said to be "frozen". The gas composition then remains unchanged. Values of KWe for different temperatures are given in Table 2.2.
(СО)х(НзО)
™ (CO)x(Ha)
Table 2.2 Temperature dependence of the water-gas equilibrium constant.
|
■Temperature (°С) |
we |
|
600 |
I0.38 |
|
700 |
*0.62 |
|
800 |
0.92 |
|
900 |
‘І27 |
|
1000 |
■1.60 |